Dopaminergic Cell Therapies for Parkinson Disease: Hope and Hurdles
Several companies are currently developing dopaminergic cell therapies for Parkinson's disease. Each one has a different approach. Will anyone succeed?
Dopaminergic cell therapies hold exciting promise in Parkinson’s disease (PD), aiming to replace the dopamine-producing neurons lost over time and address the motor deficits that define the disease. While these cutting-edge approaches offer potential breakthroughs, they face significant hurdles before they can be safely and widely available.
In this post, I’ll explore the current dopaminergic cell therapies in the pipeline, review the available clinical trial data, and address the unanswered questions these therapies bring.
Why Dopamine Replacement?
Motor symptoms in PD —such as tremors, rigidity, and balance impairment—are primarily caused by the loss of dopamine-producing neurons in the brain’s substantia nigra. By the time these symptoms become noticeable and a diagnosis is made, a staggering 60-80% of these dopaminergic neurons have already been lost.
Current symptomatic treatments are based on this principle: levodopa is a dopamine precursor (the former converts to the latter by the action of the enzyme DOPA-decarboxylase, aka AADC), effectively replenishing dopamine levels, while dopamine agonists mimic its function by stimulating dopaminergic receptors. These treatments provide excellent symptom control for many patients, particularly those in the early stages of PD.
However, as the disease progresses, the response to levodopa diminishes, requiring higher doses (sometimes up to eight times a day), which can lead to levodopa-induced complications like dyskinesias. At this stage, patients will need additional adjunctive oral therapies (sometimes taking up to four different types of medications), deep brain stimulation (DBS) surgery, or continuous duodenal or subcutaneous infusions of levodopa to maintain symptom control.
Cell-based therapies aim to address these limitations by replacing the lost neurons, potentially offering a more sustainable dopamine source for patients with advanced PD who require a more consistent dopamine release.
The concept behind dopaminergic cell therapy is that single implantation of dopamine-producing cells could restore the brain’s depleted dopamine levels, theoretically providing a continuous supply to alleviate or even eliminate the motor symptoms of PD.
Dopamine Cell Replacement in PD is not New
The concept of using dopaminergic cell transplants for PD goes back to the late 1990s and early 2000s. At that time, a pioneering clinical trial transplated embryonic dopamine-producing cells into the brains of 20 patients with advanced PD, while another 20 underwent a sham procedure. The results were published in The New England Journal of Medicine.
The cell implants involved an invasive surgical procedure. Surgeons drilled two holes in the patients' skulls and used long needles to inject dopaminergic cells, sourced from aborted embryos, deep into the brain's putamen (shown in the figure below).
While the grafts survived and produced dopamine (as measured by fluorodopa PET and confirmed in 2 patients who died due to causes unrelated to the procedure), clinical benefits were inconsistent. Some younger patients saw improvements, but older patients did not, and many developed dystonia and dyskinesia, symptoms of excessive dopamine release. Due to these mixed results, the program was discontinued.
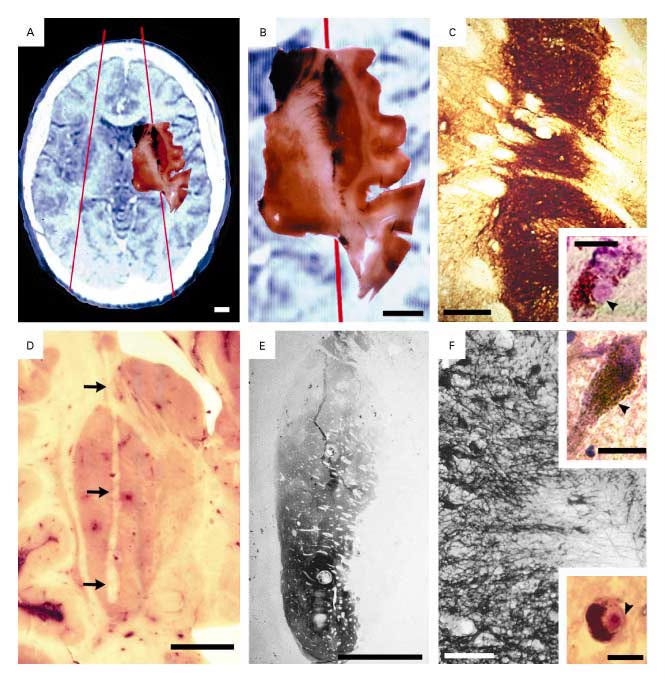
Crucially, autopsies performed years later revealed a surprising outcome: the transplanted cells, once thought to be free from disease, had themselves developed alpha-synuclein aggregates—the pathological hallmark of PD. In addition, there was significantly increased neuroinflammatory markers, suggesting, perhaps, an immune response against the grafts.
These finding underscored a vital lesson: even newly implanted cells are not immune to the ongoing neurodegenerative process of PD, highlighting the potential limitations of these therapies as purely symptomatic treatments.
Next-Generation Cell Therapies
It soon became clear that embryonic or fetal cell–based therapies for PD faced significant challenges. Ethical concerns regarding the use of aborted embryos, difficulties in producing a consistent and defined cell product, and the potential for inducing immune responses made these therapies impractical.
By the 2010s, a breakthrough came with the advent of induced pluripotent stem cells (iPSCs). This technology allows researchers to take fibroblasts from patients, de-differentiate them into iPSCs, and then re-differentiate them into virtually any cell type, including dopaminergic neurons. The advantage of iPSCs is twofold: they bypass ethical concerns and eliminate the risk of immune rejection, as the cells are derived from the patient’s own body.
In 2020, the New England Journal of Medicine published the first report of personalized iPSC-derived dopamine cells being transplanted into a PD patient. The grafts produced dopamine, and clinical measures suggested symptom improvement after 18 to 24 months post-surgery. While this was promising, it was just one patient.
Nevertheless, this result reignited enthusiasm for dopaminergic cell therapies (including BlueRock Therapeutics, NovoNordisk, Aspen Neuroscience and Sumitomo Pharma), spurring several companies to develop these treatments. Let’s take a closer look at them.
Dopaminergic Cell Therapies in the Pipeline
Many companies are developing vague "stem cell therapies" that are administered intravenously or intrathecally, often with no clear mechanism of action. These therapies lack specificity, and their actual impact on PD remains uncertain.
In this post, however, I will focus exclusively on dopaminergic cell therapies surgically implanted into the brain's deep nuclei, targeting the specific goal of releasing dopamine to alleviate motor PD symptoms like tremors, rigidity, and bradykinesia.
Currently, there are 5 ongoing clinical development programs as summarized in the following table.
BlueRock Therapeutics
BlueRock’s journey into dopaminergic cell therapy began with the discovery that embryonic stem cells (obtained from embryos created with in-vitro fertilization), much like adult fibroblasts, can be multiplied and reprogrammed into dopamine-producing neurons.
The foundational work on developing these dopamine cells, as well as the preclinical studies in animal models, was led by Lorenz Studer at Memorial Sloan Kettering Cancer Center in New York. In 2016, Studer became a scientific co-founder of BlueRock Therapeutics, which was initially launched as a joint venture between Bayer, a major pharmaceutical company, and Versant Ventures, a prominent investment firm.
In 2019, Bayer fully acquired BlueRock in a deal that valued the company at approximately $1 billion, reflecting the high expectations for its cell therapy pipeline.
In August 2023, BlueRock reported results from a Phase 1 trial involving 12 patients with PD. This open-label, non-randomized study tested two different doses of bemdaneprocel (aka BRT-DA01, aka MSK-DA01), their embryonic stem cell-derived dopaminergic cells, transplanted into the putamen of 12 patients with PD (NCT04802733). Cohort A (5 patients) received 0.9 million cells per putamen, while Cohort B (7 patients) received 2.7 million cells per putamen.
Patients tolerated the transplant well, though some experienced serious adverse events related to the surgical procedure, not the cell therapy itself.
Immunosuppressive drugs were required for a year to prevent rejection of the transplanted cells.
Crucially, fluorodopa PET scans confirmed the survival of the transplanted cells and dopamine production. The cells were still alive after 18 months, when the immunosuppression had been discontinued.
Clinically, patients in the higher dose cohort (Cohort B) showed better outcomes.
They experienced a median increase of 2.16 hours in the "ON" state (where symptoms are well controlled) and a reduction of 1.91 hours in the "OFF" state (where symptoms worsen) without dyskinesias.
Motor improvements, measured by the MDS-UPDRS-3 scale, showed a median reduction of 13 points in the high-dose group. The low-dose group saw a smaller benefit, with only a 0.72-hour improvement in "ON" time and a median increase (worsening) of 1 point in MDS-UPDRS-3.
These results sound fantastic, right? Keep in mind, this was not a placebo-controlled study, meaning patients knew they were receiving the actual treatment, and those in the higher dose group were aware they were getting the stronger dose. The lack of blinding significantly increases the possibility of bias, particularly in a disease like PD, where the placebo effect can be substantial. I'll explain more about the implications of this and the placebo effect in PD below.
Based on these results, BlueRock expects to begin enrolling patients in a Phase 2 trial by late 2024.
Lund STEM-PD
An academic-initiated trial led by Lund University (Sweden) in collaboration with Cambridge University (UK) and funded by public entities and Novo Nordisk is aiming to treat 8 patients with PD by injecting dopaminergic cells derived from embryonic stem cells (STEM-PD - NCT05635409) in an open label fashion. The trial consists of a low-dose cohort (4 patients) and a high-dose cohort (4 patients).
This approach builds on lessons learned from a previous open-label study (TRANSNEURO - NCT01898390) involving 13 patients, conducted by Cambridge University, which used human fetal ventral mesencephalic tissue. The results of the TRANSNEURO trial have yet to be published, but these, as well as preclinical studies, have informed the design of the STEM-PD trial.
Like BlueRock’s therapy, STEM-PD requires immunosuppression.
Thus far, no safety or tolerability issues have been reported in the low-dose cohort STEM-PD, and the first patient in the high-dose group has now been treated.
Aspen Neuroscience
Aspen Neuroscience, a California-based biotech that had raised US$70 million in Series A funding, has launched a groundbreaking Phase 1 open-label trial (ASPIRO - NCT06344026) to study the safety and tolerability of autologous cell transplantation therapy for PD (referred to as ANPD001).
Aspen’s added value lies in the autologous part—using their proprietary technology, Aspen transforms a person’s skin cells into dopamine neurons in the lab, which are then transplanted into the brain. This personalized approach offers two major advantages compared to BlueRock and the STEM-PD approaches:
First, it significantly reduces the risk of immune rejection, a common issue with transplants, because the cells come from the patient. Moreover, no immunosuppression is needed.
Second, it eliminates the ethical concerns associated with the use of embryonic cells.
Aspen has started screening patients for the trial, which will assess the safety and tolerability of this innovative therapy.
We should have some results by late-2025. If successful, it could represent a huge leap forward for personalized PD treatments.
Sumitomo Pharma
In 2018, the Center for iPSC Research Application (CIRA) at Kyoto University launched a Phase 1/2 open-label trial aimed at evaluating the safety and efficacy of transplanting allogenic human iPSC-derived dopaminergic progenitors (CT1-DAP001) into the brains of patients with PD. Given that these are allogeneic (donor-derived) cells, patients require immunosuppressive drugs to prevent the immune system from rejecting the transplanted cells.
Sumitomo Pharma, which is responsible for producing the dopaminergic progenitor cells, has partnered with CIRA to fund this study. The initial cohort of Japanese patients demonstrated a promising safety profile, with no significant adverse reactions.
Encouraged by these results, the trial was expanded internationally to include the U.S., where the University of California San Diego (UCSD) started enrolling patients in 2024 (NCT06482268).
Other ongoing & concluded trials
In early 2024, Chinese biotech company iRegene launched an open-label Phase 1/2 trial (NCT06167681) using "chemically induced allogenic dopaminergic cells" in 40 patients with mid- to late-stage PD. In a recent press release, the company reported that the treatment showed no significant safety or tolerability concerns and claimed improvements in motor symptoms as measured by the MDS-UPDRS-3 scale, although detailed data has yet to be released. iRegene also announced that they received FDA authorization to start dosing U.S. patients.
Several other dopaminergic stem cell trials have been conducted, though the lack of publicly available results might hint that the outcomes were less than stellar. For example, an open-label trial in 50 patients sponsored by the Chinese Academy of Sciences (NCT03119636) used human embryonic stem cell-derived dopaminergic cells. Similarly, Chinese company Allife sponsored an open-label trial (NCT03815071) in 10 patients using autologous neural stem cells, but no significant updates have been shared. Another effort in Australia, sponsored by the International Stem Cell Corporation, disclosed some results via a press release, leaving much of the data still under wraps.
This radio silence raises questions about the real impact of these therapies, with many wondering if the results didn't meet expectations.
Ongoing Challenges of Dopaminergic Cell Therapies
Placebo Response: So far, all conducted and ongoing trials have been open-label, with no placebo arm. This is crucial because patients with PD are known to have a strong placebo effect. In fact, PD patients often experience significant improvement in motor symptoms after taking a placebo, and this placebo effect is accompanied by dopamine release in the brain, which can be measured with brain PET. Studies have even shown that the magnitude of the placebo effect correlates with the perceived high cost and advanced nature of the treatment.
What could be more cutting-edge and expensive than an intraputaminal stereotactic injection of stem-cell-derived dopaminergic cells?
This combination of perceived complexity and cost sets the stage for an incredibly potent placebo response in PD patients receiving cell therapies.
Is Cell Therapy Better Than Current Treatments? For me, this is the most pressing question.
Even if cell therapies show a real benefit beyond the placebo effect, the big question is whether this highly invasive procedure is superior to standard treatments that are proven to work like medication or DBS.
"Better" in this context means both safer and more effective. The jury is still out. While the potential to restore lost dopamine is enticing, the surgical complications are a serious concern. Implanting these cells deep in the brain carries risks like bleeding, infection, and even brain damage. Additionally, transplanted cells might release too much or too little dopamine, leading to dyskinesia (involuntary movements) that can’t be easily regulated—unlike medications or DBS, where doses can be adjusted.
An additional question is whether regulators or payors could require a head-to-head clinical trial to determine the superiority of dopaminergic cell therapies - which would make clinical development much more challenging.
Immunosuppression Challenges: Many therapies use allogeneic cells (from donors or embryos), requiring patients to take immunosuppressive drugs to prevent rejection. For the older and often fragile PD population, this is a significant burden. Autologous stem cells, derived from the patient’s own body (like the ones developed by Aspen), could solve this problem, but scalability and cost could be major hurdles.
Durability of Effect: Another uncertainty is how long these transplanted cells will keep functioning. BlueRock showed that the implanted cells are still functional after 18 months, but we need longer-term data—5 to 10 years—to know whether repeat surgeries will be necessary to maintain dopamine production.
Ethical Considerations: Embryonic-derived therapies pose another challenge. As embryos are human beings, many people consider abortion wrong. In many geographies, research with aborted fetuses is not legal. All these are significant hurdles to the use of embryonic cells, which make non-embryonic cell therapies much more preferable.
Conclusions
The preliminary results of many clinical trials using dopaminergic cell therapies in PD suggest exciting possibilities but also present significant challenges. From safety concerns and immunosuppression to the durability of effects and the potential for placebo responses, these therapies are far from straightforward solutions. Moreover, dopaminergic cell therapies would provide symptomatic benefit only, with no impact on the underlying neurodegenerative process.
While they aim to replace dopamine-producing neurons lost in PD, the road to widespread clinical use is still uncertain. Will these therapies be able to overcome their current limitations and outperform existing treatments like levodopa or DBS?
Only time, further sham-controlled trials, and long-term data will tell.
Readers - Did I miss any ongoing clinical trial using dopaminergic cell therapies for PD? How do you think some of these challenges could be solved? Please share your thoughts, questions, and experiences in the comments below!
Thanks for reading. If you found this entry interesting, please, share it so that others can receive more like this in their inbox.
Views expressed here are my own and not necessarily those of my employer. All data mentioned and discussed are publicly available.