GLP-1 Receptor Agonists for Parkinson’s Disease: Promise or Mirage?
The recent failure of the Exenatide-PD3 trial forces a reevaluation of incretin-based therapies in Parkinson’s disease. Are these drugs still promising, or was the hype misplaced?
In addition to their dramatic effects on blood sugar control and weight loss, GLP-1 receptor agonists (GLP-1 RAs), the class of drugs including multi-billion blockbusters such as semaglutide (Ozempic) and tirzepatide (Mounjaro, Zepbound), have shown intriguing benefits beyond metabolism. From reducing cardiovascular risks to possibly improving inflammation and cognitive function, these drugs have sparked a wave of enthusiasm about their potential to treat a broad range of chronic diseases.
Among the most tantalizing possibilities? Neuroprotection.
For years, the idea that GLP-1 RAs might slow the progression of Parkinson’s disease (PD) has captivated both researchers and patients.
It wasn’t just theoretical: these drugs appeared to exert neuroprotective effects in animal models of PD, and epidemiological, nationwide studies suggested that people taking them had a lower incidence of Parkinson’s. The biological plausibility was strong—GLP-1 RAs reduce inflammation, improve mitochondrial function, and may modulate pathways involved in neuronal survival.
Then came the early clinical trials—small, but promising. The hints of benefit were enough to spark real hope.
But now, the tide is turning.
The long-anticipated Exenatide-PD3 trial—a rigorous, well-powered study—delivered a sobering result: no meaningful disease-modifying effect in treated patients.
Before that, a similarly well-designed trial of a pegylated exenatide analog (NLY01) met the same disappointing end.
So what went wrong? Did the early studies overpromise? Were we misled by a placebo effect? Are GLP-1 RAs merely offering symptomatic relief? Or, is there still a signal buried in the data, waiting to be understood?
Why The Allure of GLP-1 RAs for Parkinson’s
The idea that GLP-1 RAs could help PD has been built on decades of research linking metabolic dysfunction to neurodegeneration.
It is well-established that patients with type 2 diabetes mellitus have an increased risk for PD, and vice versa, patients with PD have an increased risk for type 2 diabetes.
Once the GLP-1RAs became available, preclinical studies in animal models suggested that these drugs did more than just regulate blood sugar. In animal models, they also reduced neuroinflammation, enhanced mitochondrial function, and promoted neuronal survival - all mechanisms involved in neurodegeneration in general and PD in particular (here is a review).
This led researchers to hypothesize that GLP-1RAs might potentially have neuroprotective effects, independently of any anti-diabetic and weight loss benefits.
Then came real-world patient data that supercharged enthusiasm. Large epidemiological studies in the UK and the US suggested that people with type 2 diabetes taking GLP-1 RAs had a lower risk of developing PD compared to those on other diabetes medications. Some studies reported up to a 30% risk reduction—a signal too strong to ignore.
It was only natural that clinical trials ensued. Initial clinical trials appeared to validate the beneficial effect of incretins, specifically:
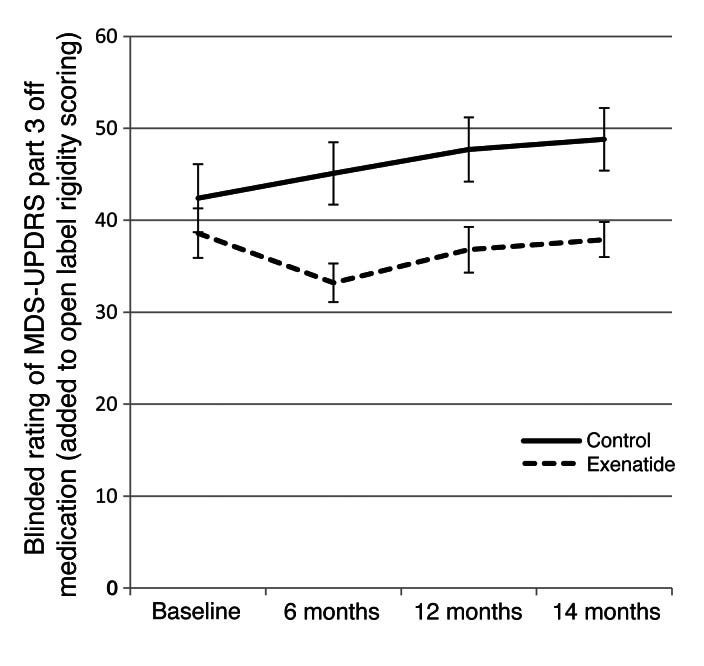
A small open-label Phase 1 study (2013) with exenatide (Byetta, twice daily, beginning with 5 μg/dose for a month and then increased to 10 μg/dose) in 45 patients with PD of moderate severity. The study was conducted in the UK. Treated patients had improvements in the MDS-UPDRS-3 scale (published here), as shown in the graph.
The MDS-UPDRS-3 is the gold-standard physician rating scale used to quantify motor deficits in patients with PD.
As with any open-label trial, this was met with interest but overall skepticism. This initial trial was followed by…
The Exenatide Phase 2 (2017) was a small, placebo-controlled trial of 62 patients with PD receiving exenatide 2 mg weekly or a placebo for 48 weeks. The results were similar to the initial trial, with statistically significant improvement in motor symptoms (MDS-UPDRS-3) vs placebo - (published in Lancet).
Another trial with once-daily self-administered injections of liraglutide (1.2 or 1.8 mg, as tolerated) or placebo in 62 patients with PD reported improvements in MDS-UPDRS-2 (an activity of daily living scale), but not in MDS-UPDRS-3, in treated patients vs placebo. This is quite surprising because, typically, you would expect the MDS-UPDRS-3 to respond better to treatment vs UPDRS-2. The full results of this trial have not been published, although there was a presentation at the 2022 American Academy of Neurology meeting.
More recently, the LixiPark trial (2023), a double-blind, randomized, placebo-controlled trial, used daily subcutaneous lixisenatide, another GLP-1 RA in 156 patients with early PD. Treated patients had significant stabilization in MDS-UPDRS-3 vs a worsening in those treated with placebo (see full publication in the NEJM).
While the difference was not dramatic (~3 points, which is well below the minimally clinically important difference in the MDS-UPDRS-3 of around ~5 points), it appeared to remain after a 2-month washout period, intended to support the idea that, because the improvements remained after removal of the incretin, the difference should be a disease-modifying, rather than a symptomatic benefit.
Despite some limitations in designs, these results raised hopes that GLP-1 RAs could be the first truly disease-modifying therapy for PD.
But were these effects real? Or just a mirage? As larger trials would soon reveal, the answer wasn’t so simple…
The Cracks Begin to Show: Negative Phase 3 Trials
For a while, it seemed like GLP-1 receptor agonists (GLP-1 RAs) were on track to become the first disease-modifying therapy for PD.
But science has a way of humbling even the most promising ideas.
Exenatide-PD3: The Highly Anticipated Phase 3 Trial
The Exenatide-PD3 trial was designed to be the definitive test. Led by researchers at University College London (UCL), it enrolled 200+ people with moderate PD and followed them for 96 weeks, measuring the progression of motor deficits with the MDS-UPDRS-3 as the primary endpoint.
The results? Disappointing (see full publication in the Lancet).
No significant difference at all (not even a trend!) in motor scores between exenatide and placebo
All the secondary endpoints were negative too.
Despite early optimism, the larger, more rigorous trial found no compelling reason to believe exenatide slows PD.
What About NYL01? Another Nail in the Coffin
Before the Exenatide-PD3 trial, Neuraly’s NYL01 (a pegylated, long-acting exenatide derivative) also failed in its large Phase 2 trial.
The trial was well designed, including levodopa-naive patients to avoid any confounding effect of dopaminergic therapies (something that the previous trials had not done).
They enrolled more than 200 patients with PD who were randomly allocated (1:1:1) to one of two active treatment groups (2.5 mg or 5 mg NLY01) or matching placebo.
After 36 weeks, NLY01 did not differ from placebo concerning change in sum scores on MDS-UPDRS parts II and III. See the graph (taken from the Lancet Neurology publication):
A subgroup analysis raised the possibility of some motor benefits in younger participants.
These back-to-back failures forced a major reality check in the field.
Why Did Larger Trials Fail After Promising Early Trials?
In early, small-scale trials, GLP-1 RAs showed promising efficacy signals in PD. But those signals all but vanished in larger, well-powered studies like Exenatide-PD3 and NLY01.
What happened? Let’s break down the possible explanations.
1. Early benefits may have been symptomatic, not disease-modifying
In the initial trials, patients on exenatide ended with better MDS-UPDRS-3 motor scores than at baseline, a surprising finding for a disease-modifying drug.
You’d expect disease-modifying therapies to slow or halt the decline, not reverse symptoms. That kind of improvement is more typical of symptomatic agents, like levodopa.
And here’s the kicker: the benefit correlated with higher baseline levodopa equivalent daily doses (LEDD). This suggests GLP-1RAs might enhance dopaminergic signaling, again pointing to a symptomatic effect. Notably, the NLY01 trial, which enrolled dopaminergic-naïve patients, was negative. See graph:
2. The observed benefits may stem from correcting underlying cardiometabolic dysfunction
One of the primary mechanisms of GLP-1 receptor agonists is the modulation of insulin metabolism. While these trials excluded patients with a formal diagnosis of type 2 diabetes, it’s highly plausible that a substantial proportion of participants had early metabolic abnormalities, such as insulin resistance, elevated fasting insulin, or even undiagnosed prediabetes.
Treatment with GLP-1 RAs in these individuals may have corrected this subtle metabolic dysfunction, improving systemic energy balance, reducing inflammation, and potentially enhancing motor function, as an indirect result. In this scenario, the clinical benefits would reflect the metabolic optimization rather than true disease modification of Parkinson’s pathology.
To test this hypothesis, it would be critical to conduct subgroup analyses based on baseline metabolic markers, such as fasting insulin, HOMA-IR, or HbA1c levels, to determine whether patients with higher metabolic risk experienced greater benefit. This could help clarify whether the therapeutic effects observed in earlier trials were primarily driven by improvements in metabolic health rather than direct neuroprotective effects.
3. Unblinding and the placebo effect
GLP-1RAs come with telltale side effects: nausea, vomiting, and -of course!- weight loss. If patients figured out they were on active drug, they may have unconsciously boosted their performance—a well-known challenge in Parkinson's disease trials.
It would be fascinating to correlate weight loss with motor improvement, but unfortunately, data on this is inconsistently reported across trials.
4. Poor brain penetration
Many GLP-1RAs barely cross the blood-brain barrier (BBB). In Exenatide-PD3, CSF levels were just ~1% of plasma, suggesting limited central penetration.
Animal studies show that semaglutide can enter the brain through circumventricular organs, which lack a BBB—but these levels are modest.

Whether that’s enough to affect human neurodegeneration remains an open question.
5. Treatment may have come too late
One possibility is that GLP-1 RAs are being administered too late in the disease course to meaningfully alter its trajectory. By the time most patients are diagnosed with PD, substantial and irreversible neurodegeneration has already occurred, particularly in the nigrostriatal pathway. At this stage, even a drug with neuroprotective potential may struggle to produce a measurable effect.
The key may lie in targeting earlier stages of disease, including individuals with prodromal PD, those with REM sleep behavior disorder, hyposmia, or subtle motor symptoms, before significant neuronal loss sets in.
Supporting this idea, subgroup analysis from the NLY01 trial suggested that younger patients experienced greater benefit compared to older participants. While this doesn’t prove earlier-stage efficacy, it does hint that the degree of neurodegeneration, or perhaps neuroplasticity, at baseline may influence the outcome.
What’s Next for GLP-1 RAs in Neurodegeneration?
The failure of the Exenatide-PD3 and NLY01 trials has cast serious doubt on whether GLP-1 RAs have a future in PD. But does this signal the end of the road?
Not necessarily.
While enthusiasm around GLP-1 RAs in PD has cooled, the broader field of neurodegeneration is still very much in play.
Novo Nordisk is currently running two large Phase 3 trials (EVOKE and EVOKE+) testing semaglutide (Ozempic, Wegovy) in Alzheimer’s disease. A positive outcome would reshape the narrative and potentially reignite interest in GLP-1 RAs across neurodegenerative disorders.
But if the field is to move forward, future trials will need to rethink both strategy and patient selection:
Target the right patients: Are those with metabolic risk factors, diabetes or prediabetes, obesity, hypertension, more likely to benefit?
Start earlier: Could GLP-1 RAs be more effective in prodromal PD, before irreversible damage occurs?
Extend duration: Were the trials simply too short to capture a true disease-modifying signal?
Mirage or Just a Setback?
The GLP-1 RA journey in PD has been turbulent: early enthusiasm, promising signals, and now sobering late-stage failures. The Exenatide-PD3 trial didn’t just miss by a hair, it challenged the entire premise.
So, while the promise of GLP-1 RAs in PD might have been an illusion, the bigger question, can GLP-1RAs alter the course of other neurodegenerative disorders?, remains open.
All eyes now turn to semaglutide’s Alzheimer’s trials. If they succeed, GLP-1 RAs may get a second chance in PD. If they fail, the field will need to pivot—either to different molecules, different patients, or perhaps a different hypothesis altogether.
One thing is clear: in neurodegenerative drug development, hype comes easy, but clinical proof is hard-won.
Readers - what are your thoughts on GLP-1 receptor agonists for Parkinson's disease? What do you think are the reasons for the discrepancy? Do you think other incretins might do better? Please share your thoughts, questions, and experiences in the comments below!
Thanks for reading. If you found this entry interesting, please, consider subscribing and sharing so that others can receive more like these in their inbox!
Views expressed here are my own and not necessarily those of my employer. The data mentioned and discussed in this Substack are publicly available.